by Roger Martyn
Summary
- Soil humus is a very valuable substance and contributor to soil fertility.
- It is a storehouse of plant nutrients and trace minerals and because of its colloidal properties it can hold three times the amount of nutrients as clay
- It helps make nutrients available to plants
- It improves soil tilth, making soil loose and crumbly. This contributes high water absorption and holding, good aeration, low water and wind erosion, and ease of ploughing and cultivation.
- It provides a very conducive environment to soil microbes and their activities
- It has valuable filtering and buffering properties to applied toxins and fertiliser excesses.
- It is a stable sink for sequestering significant amounts of greenhouse gas causing carbon.March 2015
Humus is amazing stuff and I think is the key component of soil fertility. A big call perhaps but read the following and make up your own mind.
The presence and quantity of humus is often mentioned in discussions on soil fertility, however there is often a poor understanding of what humus actually is and what it can contribute to soil fertility. Accordingly, many opportunities to enhance soil fertility or even just maintain it are missed.
The best way to understand what humus has to offer is to start with an explanation of what humus actually is.
Humus is found in the soil, predominantly within the rooting zone of the topsoil. Humus is part of the soil's organic matter. In biological terms, organic matter is anything that is living, or was once living. Soil organic matter therefore includes any living matter such as plant roots, bacteria, nematodes, fungi, earthworms, or dead matter of the same including their excreta. Organic matter also includes humus itself.
It is also very helpful to understand what organic matter is in chemical terms. Chemically, 'organic matter' means any substance that has carbon-carbon and carbon-hydrogen bonds as its chemical basis. Such chemistry is referred to as 'organic chemistry'.
Virtually all the chemistry of plants and animals is organic chemistry. A carbon atom has the ability to bond with itself and up to three other atoms. This allows carbon to form chains or rings of carbon with itself and also bond with other carbon chains as well as a multitude of other elements. It is these attributes of carbon that facilitate the chemical diversity and complexity that makes life even possible.
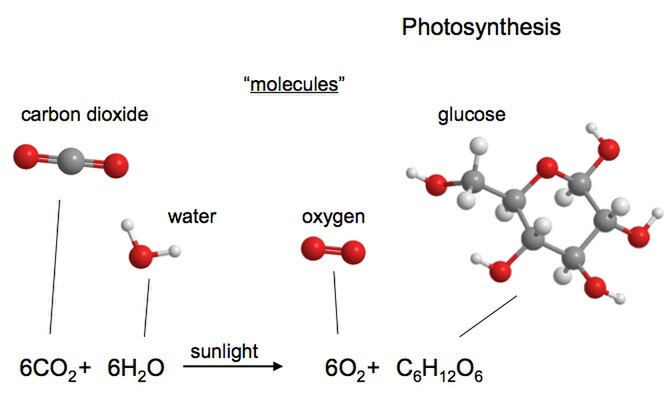
Most of us are probably quite unaware of just how complex some of the organic compounds in plants and animals are. Even a relatively simple plant protein molecule might typically consist of 500,000 atoms. Yet such complexity all starts from the relatively simple beginnings, from the production of sugars from photosynthesis. Sugar (glucose) produced from photosynthesis contains just 24 atoms i.e. C6H12O6.
Photosynthesis takes place in plants in organelles called chloroplasts which are found in the cells of leaves and stems. Chlorophyll (the stuff that makes plants appear green) in the chloroplasts is able to utilise the energy of the sun to chemically combine carbon dioxide (CO2) from the air with water (H2O) taken up by plant to form the simple sugars. Oxygen is given off as a by-product.
As this simple illustration show, the plant sugar glucose is 'organic' in that it consists of a very simple chain and ring of carbon atoms with oxygen and hydrogen atoms attached.
Chemical processes in other parts of the plant cells can process and construct these simple sugars into even more complex organic compounds, such as amino-acids, proteins, starches and hormones through the addition of elements such as nitrogen and sulphur . These processes are orchestrated via chemical instructions initiated by the genetic code of DNA, itself also an organic compound, and one that is found within the nucleus of every living cell. These organic compounds can take on very different forms throughout the plant to form for example rigid structures such as wood and leaves or in soluble forms such as food stores (starches and sugars), or as hormones, enzymes or anything else required for a plant to live.
Animals are able to extract nutrition from organic compounds through the eating and digestion of plants or as in the case of carnivores, through the eating and digestion of other animals. Digestion essentially involves the breakdown or deconstruction of the organic compounds that plants or animals consist of into simpler compounds. WThese simpler compounds can in turn be used as raw materials for the construction oforganic compounds required by the plant or animals consumers own life processes. Some of organic matter breakdown results in significant amounts of CO2 being released to the air, simply a result of normal cell respiratory processes being carried out by plants and animals. For many microbes this might involve CO2 diffusing through their cell walls into the surrounding airspace. WBy contrast, higher level plants and animals have more sophisticated respiratory mechanisms such as leaf stomata in the case of plants, or respiratory spiracles or lungs in the case of animals. While such CO2 is derived from organic matter, it is not part of humus.
When plants lose their leaves or completely die or when animals excrete waste or die, the soil is presented with large amounts of organic matter of considerable chemical complexity. However, since this 'dead' organic matter is no longer part of something living, the defence processes a living plant or animal has to guard against cell degeneration and decay will no longer be active making 'dead' organic matter much more susceptible to decomposition .
Decomposition of 'dead' organic matter includes natural oxidation processes from oxygen in the air . However the vast bulk of decomposition in a healthy and active biological ecosystem is carried out in the soil due to the activities of soil macro-organisms such as earthworms and woodlice in conjuction with soil micro-organisms such bacteria, yeast and fungi, with microorganisms making by far the greatest contribution. . The biological processes can be very efficient in breaking down organic matter. this is because a healthy soil typically has a great diversity of organisms that have evolved to take advantage of almost all the energy and nutrient opportunities that the decomposition of dead organic matter potentially presents. Such is the diversity and specialisation that when one lot of organisms have decomposed the organic matter as far as they can, there will be another species present that can takeover and further decompose what is left and so on until what remains can for all intents and purposes, be decomposed no further.
This final product that cannot be broken down any further is in essence 'humus'. Humus is chemically complex and eclectic in its make up consisting of all manner of broken bits and pieces of organic carbon chains, rings, and associated elements and compounds. Humus is therefore essentially the chemical residues of what might once have been functioning organic chemical compounds such as cellulose, protein, starches, hormones, enzymes, RNA and DNA . Humus can therefore be very rich in different minerals and elements, it will also be rich in a vast array of partially formed chemical compounds of incredible diversity meaning no two lots of Humus can be alike. While humus is what is left after decomposition can proceed no further, it it is a potentially rich source of nutrients, while its physical and chemical attributes can contribute greatly to soil fertility as will be explained.
As a result of decomposition in humification of organic matter, much of the chemical residues making up humus will have atoms and molecules that are chemically, only partially bonded. This is especially true for carbon and the many compounds that carbon can form as previously described.. The many broken chemical bonds that end up in what is humus mean that that there will be many unbalanced negative charged molecules, (anions) present. And since humus has a lot of carbon in it, there is likely to many negative charges present - given each carbon atom potentially has four negative sites.
Chemically, negative charges like to be balanced or neutralised with positive charges. Chemical substances with a nett positive charge are usually referred to as cations.
We can therefore describe extent of the nett negative charges arising from all these unbalanced carbon bonds in humus, as its 'Cation Exchange Capacity' or 'CEC' ie its capacity to attract (and exchange with) substances with a positive charges (cations) – represented by a + as for example with Magnesium - Mg+).
The CEC of a substance is a measurable quantity and can be ascertained for a soil using a standardised laboratory test. The reason why we would want to know the CEC value of a soil is because it has profound implications regarding its fertility. And the word 'exchange' in the term 'Cation Exchange Capacity' gives us an insight as to why this is so.
Since many of the cations are plant nutrients and animal nutrients (K+, Ca+, Mg+ Cu++, Zn++ etc) it is easy to see that humus has the potential to attract and hold such nutrients in a soil – which in fact is the case as will be explained.
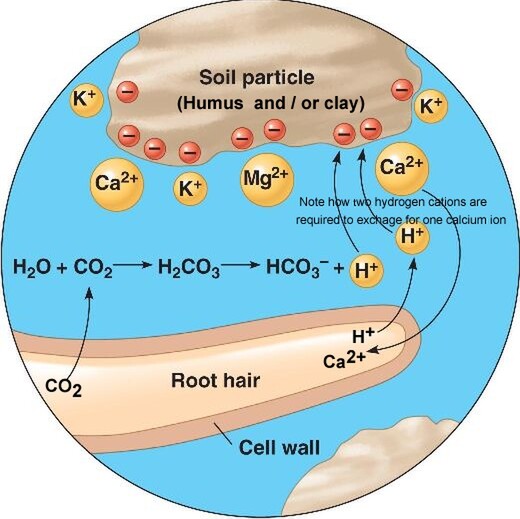
As already explained, a soil that has a high CEC value will attract cations (molecules with a positive charge) and bond them to negatively charged sites in order to be chemically balanced. Since many of these cations are also plant nutrients, this means a soil with a high CEC has the potential to attract and hold a lot of plant nutrients. Fortunately these bonds are sufficiently strong enough not to be broken by the dissolving properties of water and so stopping potential plant fertility from being washed down past the root zone. However, a plant root is able to access these cations by actively exchanging a hydrogen cation for the cation nutrient it needs. Hence the term 'Cation Exchange'. Mechanisms by which a plant can do this isby giving off carbon dioxide (CO2) from it root hairs, a result of normal cellular respiration or by actively exuding mildly acidic substances from its root hairs that are rich in hydrogen cations (H+). As shown in the illustration below, root hair emitted CO2 combines with water in the soil solution to form carbonic acid (HCO3) plus a free H+ cation. This combined with H+ cations exuded from root hairs can then swap places or exchange with other cations bonded with the soil by CEC forces. The cations displaced by this process are then available for plant uptake as nutrients.
Examples of nutrient cations are salt forms of calcium, sodium, magnesium, copper, and zinc and most other metals. In their salt forms cations carry a nett positive charge and are represented notationally as Na+, K+, Ca++, Mg ++, Cu++ Zn++ etc. These nutrients might be present in the soil solution already as soluble salts, or present as part of soil minerals and ores, but become soluble in the soil solution due to the actions of weathering, or chemical actions of soil microbes and plant exudates.
Cation exchange in soil.
Clay soils and humus and bind positively charged minerals (cations such as Ca2+).
Hydrogen ions (H+) help make nutrients available by displacing the cations.
Plants secreting H+ by cellular respiration: CO2 reacts with H2O to form carbonic acid (H2CO3) in the soil, which dissociates to add H+ to the soil.
As shown in the above illustration, the number of H+ cations swapped depend on their charge. For example two H+ cations are required to swap for one Calcium (Ca2+) cation.
It is to be noted that all fractions of a soil sand, silt and clay, will have CEC characteristics of some extent, with sand having typically low values, silt somewhat higher and clay higher still.
While CEC values for clay are appreciably high, weight for weight, they will normally be nowhere as high as they are for humus. Humus CEC levels are in typically so high that an application of even a couple of millimetres of quality compost with a high humus content will when applied to a soil, significantly improve its overall fertility characteristics. It should be noted that while humus can be a part of a soil, it is not part of the clay, silt, sand or rock fractions even though it can mix and combine freely with them.
The sand, silt and clay fractions are inorganic since chemically, their structure are not based on carbon-carbon and carbon-hydrogen bonds. A soil doesn't need humus to be considered fertile even though a soil with humus is likely to have significantly more desirable fertility characteristics.
It should also be pointed out that a soil with high CEC values is not necessarily high in fertility.
To understand this, we need to appreciate that the CEC value of a soil is an indication of it capacity to hold exchangeable cations, but not an indicator of the actual amount of exchangeable cations it actually holds. For that we have to know the 'base saturation' values which indicate the amount of Cation Exchange Capacity that is 'filled'. Base saturation figures are often expressed in percentages, both individually for the major cations like Ca, Mg, K, and Na, and as a total of all cations – the Total Base Saturation% value. This part of soil chemistry is beyond the scope of this article but it is sufficient to say that a soil with a high CEC but low total base saturation value has the potential to exhibit high fertility characteristics, by virtue of its high Cation Exchange Capacity, but if its base saturation levels are measuring low, its current fertility will also be low.
Humus itself is likely to have good levels of nutrient (ie high base saturation levels) and also a wide variety of different nutrients. This is hardly surprising once we remember that humus is essentially the broken down materials of what was once living organic matter jam packed with nutrient. This fact combined with its high CEC characteristic means humus is potentially a good source of many different nutrients and weight for weight, likely to provide a great 'holding tank' for nutrients, which for most instances, is the case. These are both great soil fertility characteristics for a soil to have meaning the addition of humus to a soil can only be a good thing to happen.
So far we have focused mostly on the chemical characteristics of humus as relates to soil fertility. There are however several physical attributes of humus that contribute greatly to soil fertility.
The first one is its sticky, resinous and tar like nature. This imparts great soil building and holding attributes. Humus has these characteristics because of its high carbon content. In this respect it shares many chemical and physical characteristics with fossil fuels such as oil, tar and lignite deposits. The dark colour of humus is a simple and useful visual indicator of potential soil fertility – the darker the soil, the higher the likely fertility – with the darkness simply a result of humus's high carbon content.
The sticky and resinous like characteristics of humus allow soil particles to crumb and remain holding together, providing soil with structure and tilth, even when subject to substantial amounts of water or wind force. This is vital to ensuring a soils ability to resist wind and water erosion. It is well understood fact that the decline in soil humus content is directly related to increased soil and wind erosion throughout all regions of the world. .
This 'sticking together' also allows the soil to drain excess water and allow the soil's flora, fauna and natural oxidation processes to 'breathe', aspects all vital to good soil fertility. The resinous nature of humus also ensures soil particles do not compact, facilitating plant roots to access soil nutrients. As plant roots or earthworms and soil insects penetrate the soil, channels left behind will remain intact due to the cement like attributes of the resins in humus. TThis results in a vastly improved air and water movement within in the soil.
These physical characteristics of humus, combined with the high CEC, allow soils to hold vastly greater amounts of water than they would otherwise. These amounts are over and above a soil’s water holding capacity attributable t to soil type -that being the relative percentages of sand, silt, and clay. The addition of humus can greatly enhance a soils water holding capacity. For example, one part of humus by weight will hold between 4 and 7 times its weight of water. That means 1gram of humus will hold between 4 and 7 grams of water. This works out at about 100,000 litres of water per hectare per % of humus in the soil. Put another way, the water holding capacity of a soil can improve by about 10 ml of rain per % of humus increased (NB: 1ml of rainfall equates to 10,000 litres per hectare of water). Given that many soils conventionally farmed over the last 50 to 100 years have lost on average 3% of their humus content, this means that such soils have on average lost the capacity to hold 300,000 litres per hectare of water they might otherwise have been able to hold. This is an extraordinary loss of land drought proofing in any term
.
Fortunately such losses are readily reversible through the adoption of some basic biological farming farming techniques. An explanation of these are beyond the scope of this article however they can be summarised as achievable simply by employing farming techniques not adverse to soil microbiology. Unfortunately this is something that majority of conventional chemical based farming techniques struggle to achieve let alone even attempt to address.
The soil building and water and nutrient holding characteristics that humus imparts to a soil provides wonderful housing for soil microbes to live and thrive in. Many of the soil microbes set up symbiotic relationships within the plant hair root zone, whereby they exchange plant nutrients for sugars exuded by plants via their root hairs. This is commonly referred to as the 'liquid carbon cycle path'. Here carbon is transferred into the soil via the sugars exuded by plant roots (remember, sugars are made up of carbon, hydrogen and oxygen – a result of photosynthesis) and these sugars are then accessed by soil microbes within the root hair zone for their energy and other biochemical requirements. Eventually these soil microbes will live, breed and die and become part of the general soil decomposition and humification process as previously described. In doing so, the liquid carbon pathway provides and alternate but very important pathway of introducing carbon to soils.
Of the plant nutrients harvested and transferred by the microbes to the plant roots in this provess, some might come directly from the humus, some from the soil itself, and some from dead organic matter if the microbes concerned are decomposers or organic matter.
Off the nutrients transferred to the plant, some will be in the form of simple elemental ions, whereas others maybe in the form of simple organic compounds such as amino acids, fragments of DNA and so on, all which will be abundant in well decomposed humus.
This can benefit the rate of plant growth since some of these organic bits and pieces will be in the form of chelated elements, that is, elements bonded with organic compounds, which in that form are much easier for a plant to uptake. Another factor is where nutrients taken up by the plant are already in the form of simple organic compounds, such an amino-acid. Here much of the 'preliminary' work has already been done before uptake by the plant making the overall process of nutrient uptake and turning these into other plant requirements a more efficient one. Much of this would be difficult without the presence of humus.
While the liquid carbon cycle arising from soil microbe-plant root symbiotic relationships is an important part of introducing carbon into the soil, the humus itself, by virtue of it being so thoroughly broken down, is itself an important aspect of soil carbon acquisition and capture. In essence, humus is a very stable form of carbon both chemically and physically. If left undisturbed in soil, it will remain in that stable state for many hundreds of years and longer. Humus is therefore considered to be an excellent 'carbon sink'. This has important implications in sequestering carbon for climate change purposes.
Each percentage increase in a soil's humus content accounts for around 60 tonnes / ha of carbon dioxide (CO2) sequestered. Given that an average passenger vehicle emits around four tonnes of CO2 a year, increasing soil humus levels clearly has a massive carbon sequestering potential. Ithas been shown that establishing and maintaining good perennial pastures is the most consistent and efficient means of raising and maintaining carbon levels.
Toxins Filter
Another very important property of humus not widely appreciated but certainly easy to understand is the ability of humus to filter out toxins and buffer the effects of nutrient excesses. Being so rich in carbon, it has wonderful filtering and buffering properties just like carbon filters do. Humus has the ability to remove and detoxify harmful things that have been applied to the soil, such as heavy metals contamination and pesticide residues. The presence of humus helps ensure these pollutants don't spill into the water tables or get taken up by plants and passed on through the food chain to accumulate in toxic amounts within higher order animals, including humans. This of course is all relative to the level of contaminants applied to the soils but the amounts removed and protection afforded has been shown to be amazingly high. A soil with a high humus content is also likely to buffer and protect plants from excess applications of nutrients. While such applications will be very hard on soil microbial life, it would be much harder both on the soil microbes and plants growing in the soil if the humus contents were low.